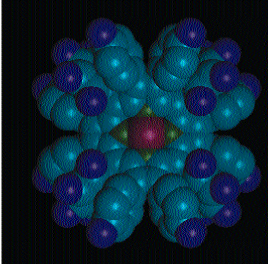
 ELECTRON DEFICIT: How many flourines (purple) can a porphyrin molecule hold? Synthesized in the laboratories of UC Davis chemistry professor Kevin Smith, this novel compound boasts 28 flourines as shown in the three-dimensional molecular structure revealed by X-ray crystallography. Known as Ni(II)F28DPP, the flourines on this mostly carbon molecule result in a kind of electronic deficit in the porphyrin ring; its special properties will be tested further. Smith's group studies porphyrins, many of which are integral components of biological processes--for example, the transport of oxygen and carbon dioxide in the body and photosynthesis in plants--and are useful in pharmaceutical applications, such as photo-dynamic therapy cancer treatment. Daniel Nurco completed the scientific research and created the artwork. Detailed descriptions and more illustrations can be found at http://www-chem.ucdavis.edu/groups/smith/index.html.
ELECTRON DEFICIT: How many flourines (purple) can a porphyrin molecule hold? Synthesized in the laboratories of UC Davis chemistry professor Kevin Smith, this novel compound boasts 28 flourines as shown in the three-dimensional molecular structure revealed by X-ray crystallography. Known as Ni(II)F28DPP, the flourines on this mostly carbon molecule result in a kind of electronic deficit in the porphyrin ring; its special properties will be tested further. Smith's group studies porphyrins, many of which are integral components of biological processes--for example, the transport of oxygen and carbon dioxide in the body and photosynthesis in plants--and are useful in pharmaceutical applications, such as photo-dynamic therapy cancer treatment. Daniel Nurco completed the scientific research and created the artwork. Detailed descriptions and more illustrations can be found at http://www-chem.ucdavis.edu/groups/smith/index.html.